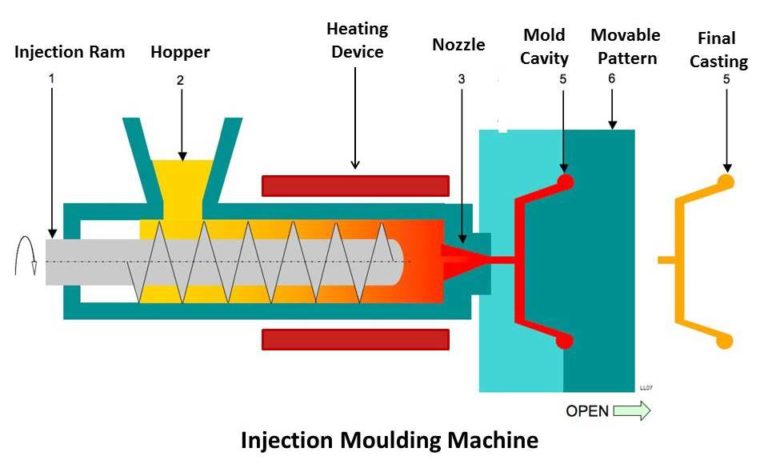
About medical injection mold
Compared with traditional injection molding, medical injection molding requires more specialized equipment. In the field of medical devices, the smallest components are often the most important in relation to the functionality and safety of the medical device. Using the best proven manufacturing processes and tools is more important than ever to the success of product development.
With advanced injection molding, complex parts in a variety of medical-grade plastics can be successfully validated for production.
One of the many advantages of this state-of-the-art facility is maximizing the efficiency of our process engineering team to develop the widest possible process window.
Advantages of Injection Molding for Medical Parts
The medical injection molding process goes beyond similar production procedures in the industry. With its smooth and seamless operation, the process offers many advantages, including but not limited to:
Multiple material options
The injection molding program offers the widest selection of materials to choose from. While medical injection molding has narrowed the range of injection molding materials, there are still many materials suitable for making medical-grade parts. We’ll discuss this more later in this guide.
Эканамічная эфектыўнасць
Medical plastic injection processes are set up in a way that helps reduce unnecessary injection molding costs—mass production deliveries and high-volume manufacturing help maximize the process. Therefore, whenever a large number of medical injection parts are produced, the injection molding process can reduce the cost per part.
Durability
A known fact about plastic used for injection molding is that it is strong and durable. These materials provide stubborn strength and resistance to adverse environments and use. As a result, the products of this process can comfortably withstand heat, blunt force and vibration without any cracking or breakage. Also, they do not succumb to high temperatures when they are sterilized in an autoclave.